When people learn about gemstones, one of the first surprises is this: carbon does not appear on the list of major elements that make up the Earth’s crust, even though carbon forms one of the most famous gems—diamond.
So what’s the truth behind this?
This absence might confuse many, considering the significance of carbon in gemology.
Let’s break it down simply and accurately.
1. The Table Shows Crust Composition — Not All Gem Elements
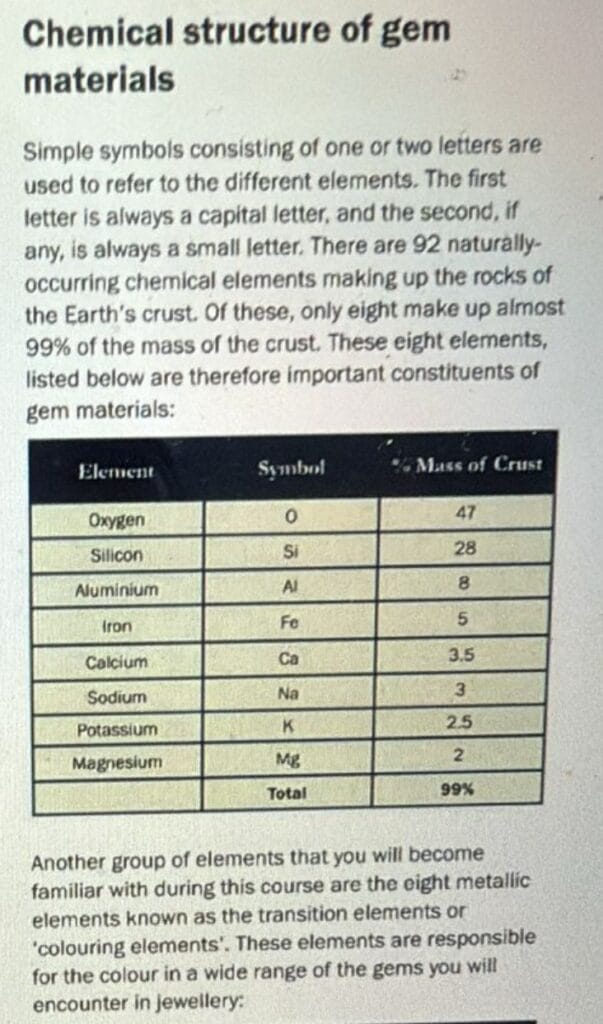
The table commonly shown in gemology classes lists the eight most abundant elements in the Earth’s crust by mass:
- Oxygen (O)
- Silicon (Si)
- Aluminium (Al)
- Iron (Fe)
- Calcium (Ca)
- Sodium (Na)
- Potassium (K)
- Magnesium (Mg)
Together, these eight elements make up more than 99% of the entire crust. Visit this link to see the most common elements
This is why they are highlighted—they form the foundation of most mineral and gem structures.
Carbon simply does not belong in this “top eight.”
2. Carbon Is Present — But Extremely Rare
Carbon does exist in the crust, but in very small amounts.
Scientific sources estimate carbon’s abundance between:
- 0.02% (Encyclopaedia Britannica)
- 0.18% (geochemical tables)
That puts carbon around the 15th most abundant element, far from the crust’s major building blocks.
When you look at your table and see “Total = 99%,” carbon is part of the remaining 1%, along with dozens of other minor elements.
This alone explains why carbon doesn’t appear.
3. Diamond Forms in the Mantle — Not the Crust
Here’s the part many people don’t realize:
Diamond is not a crustal mineral.
It forms at depths of 140–200 km inside the Earth’s mantle, under extreme pressure and temperature.
Only violent eruptions—kimberlite or lamproite pipes—bring diamonds up into the crust.
So even though diamond is precious and famous, it does not contribute meaningfully to the chemical makeup of the crust.
That’s why carbon never appears in “crust abundance” tables.
4. Why This Matters for Gemology
Most gemstones we encounter—ruby, sapphire, emerald, topaz, garnet, peridot, quartz—are built from the eight major crustal elements.
That’s why gemology emphasizes:
- Silicates (minerals with Si + O)
- Oxides (minerals with metal + O)
- Aluminium-bearing minerals (like corundum)
- Iron-bearing minerals (like garnet and spinel)
Diamond is the exception, not the rule.
Understanding this helps students see:
- Why silicate minerals dominate gemology
- Why oxygen is by far the most common element in gemstones
- Why carbon-rich gems are rare and valuable
- Why the Earth produces far more quartz than diamond
5. In Summary
Carbon isn’t listed because:
- It’s not one of the eight most abundant elements in the Earth’s crust
- It makes up only 0.02–0.18% of crustal mass
- Diamond is a mantle mineral, not part of crust chemistry
- Most gemstones form from silicates and oxides—built from the major eight elements
This is a great teaching moment:
The rarity of carbon in the crust explains the rarity—and value—of diamond.
Want to Learn More at The Gem Museum?
At The Gem Museum, we help visitors understand how Earth’s chemistry shapes the gems we love. Whether you’re a student, jeweller, collector, or enthusiast, our guided tours make gemology simple, scientific, and inspiring.
Visit us at 9 Perak Road, Singapore and experience the world of gems up close.
Book your visit here! https://www.thegemmuseum.eventbrite.com